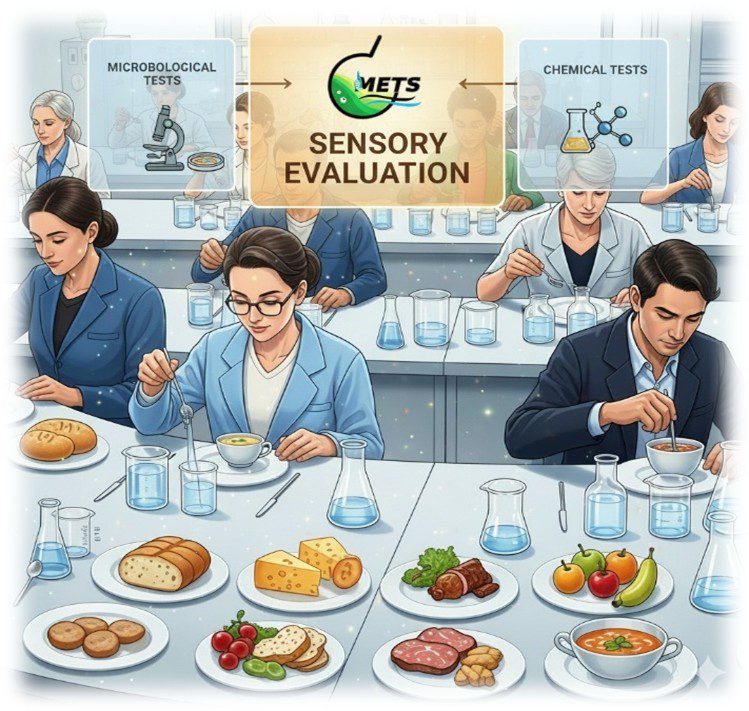
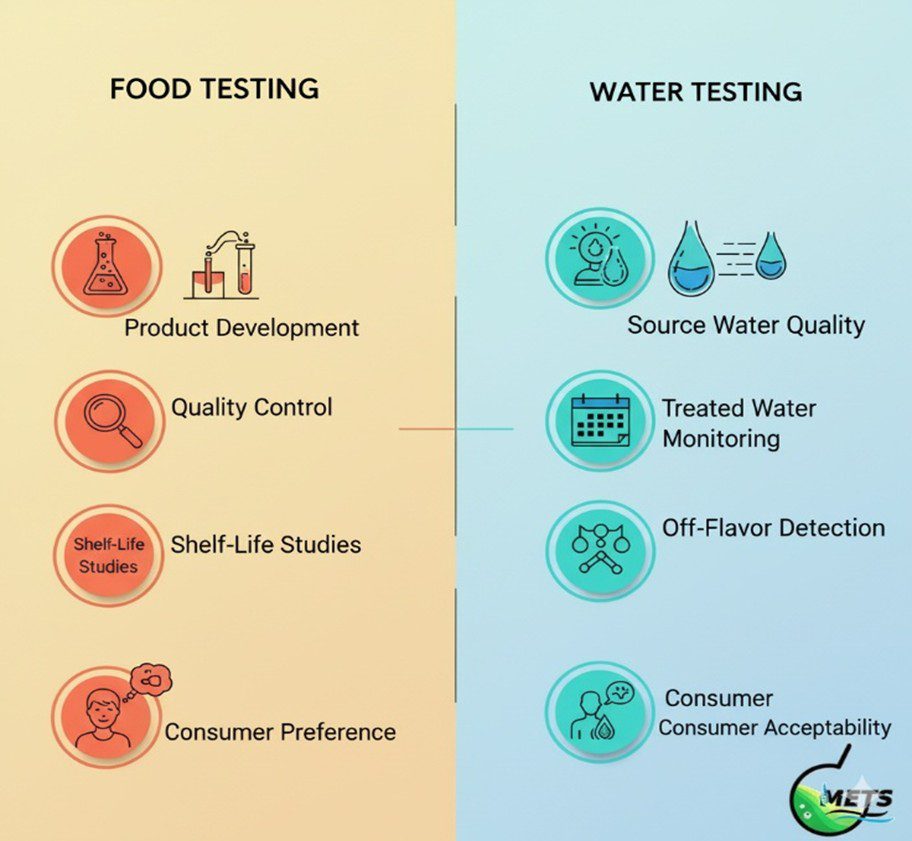
Middle East Testing Services (METS Lab) is your trusted partner for comprehensive sensory evaluation services for food and water. We assess critical sensory attributes such as taste, odor, color, texture, clarity, and overall acceptability. Our ISO/IEC 17025-accredited laboratory employs expert panels and standardized protocols aligned with UAE.S, ISO, WHO, GSO, BS, FSSAI and other international benchmarks.
Whether your focus is product development, reformulation, or quality control, METS Lab supports food and beverage manufacturers, water utilities, retailers, and regulatory bodies across the Middle East. Our services ensure that every product not only meets safety and regulatory standards but also delights consumers. With fast turnaround times, meticulous data interpretation, and a commitment to precision, we help your brand build trust, stand out in the market, and achieve sensory excellence.

-Why Sensory Evaluation is Essential in the Food & Water Industry-
At METS Lab, we believe that sensory evaluation is more than a test—it’s a critical tool to understand how consumers truly experience your products. While laboratory tests confirm safety and compliance, sensory evaluation captures the real-world perception of taste, aroma, texture, and appearance. This insight helps brands consistently deliver products that delight.
Sensory evaluation engages the five primary senses—sight, smell, taste, touch, and even hearing—to identify subtle quality attributes that can influence consumer satisfaction:
- Appearance: Visual appeal signals quality at first glance. Black spots on brinjal, for example, may indicate spoilage or insect damage.
- Color: Reflects freshness and ripeness—bright yellow bananas, deep red tomatoes, golden mangoes.
- Flavor: A combination of taste, aroma, and mouthfeel, flavor defines a product’s memorability.
- Odor (Aroma): Volatile compounds create aroma. Improperly stored spices may lose their signature fragrance, affecting sensory experience.
- Taste: Registered by taste buds, taste drives consumer preference—sweet cherries are often preferred over sour ones.
- Mouthfeel: Texture and physical sensations—crispness, crunchiness, softness, stickiness—play a vital role. A crisp wafer is far more appealing than a soggy one.
Unlike conventional laboratory tests, sensory evaluation doesn’t require complex equipment, is cost-effective, rapid, and provides actionable insights that help food manufacturers:
- Refine and optimize product formulations
- Ensure consistent quality and consumer satisfaction
- Protect brand reputation and reduce product complaints
At METS Lab, our expert sensory panels simulate real consumer experiences, bridging the gap between lab results and market success. We ensure your products are not just safe, but truly enjoyable.

-The METS Lab Advantage: Sensory Evaluation Services-
METS Lab is a premier provider of sensory evaluation services for food and water, leveraging trained panels and globally recognized methodologies to assess flavor, aroma, appearance, and texture.
Key offerings include:
- Water and Food Testing: Evaluating taste, smell, clarity, mouthfeel, and overall acceptability.
- Expert Human Panels: Combining trained assessors with strict protocols to deliver precise, reliable data.
- Comprehensive Insights: Helping brands uphold quality, meet regulatory requirements, and build consumer trust.
-What is Sensory Evaluation and Why It’s Critical? –
Sensory evaluation is the scientific analysis of how human senses perceive food and water. It measures attributes such as flavor, aroma, appearance, texture, and mouthfeel under controlled conditions.
As consumer expectations rise for consistency, freshness, and sensory appeal, sensory evaluation becomes indispensable at every stage of the product lifecycle—whether developing new products, implementing reformulations, monitoring shelf life, or ensuring batch-to-batch quality.
-International Standards for Sensory Evaluation-
Sensory evaluation is governed by international standards – for example, ISO 8586:2023 (Selection and Training of Sensory Assessors) defines how to select and train human assessors; ISO 13301:2018 deals with sensory analysis methodology and thresholds, ISO 8589 (Design of Sensory Analysis Rooms). These standards help ensure your sensory data is robust, consistent, and defensible.
-Benefits of Sensory Evaluation-
Industries benefiting from sensory analysis include: food & beverage manufacturing, water utilities, packaging, retail, R&D, and regulatory authorities. Key benefits include:
- Ensure product quality and consistency, so every lot reflects your intended sensory profile.
- Align product design with consumer preferences, enhancing acceptance and market success.
- Monitor shelf life and storage impact — understanding how sensory qualities change over time.
- Meet regulatory or customer-driven requirements for sensory attributes (absence of off-odors, clarity, flavour consistency, etc.).
- Protect brand reputation by minimizing sensory defects or negative consumer feedback.
-Role of a Testing Laboratory in Sensory Evaluation-
Sensory evaluation laboratories, like METS Lab, ensure products meet quality expectations and consumer satisfaction. Leveraging standards such as ISO 8586, ISO 13299, and ISO 8589, METS Lab provides precise, reproducible, and actionable insights.
Core Laboratory Roles Include:
- Panelist Selection, Training & Monitoring: Recruiting skilled assessors, training them, calibrating performance, and ensuring consistency.
- Method Development & Protocol Design: Defining attributes, test types (discrimination, descriptive profiling, hedonic), sample preparation, serving order, and randomization.
- Controlled Testing Environment: Sensory booths, neutral lighting, odor control, stable temperature, palate cleansers, and blind/coded samples.
- Executing Sensory Tests: Discrimination tests (triangle, duo-trio), descriptive profiling, consumer/acceptability tests.
- Data Collection, Analysis & Reporting: Statistical design, analysis of panelist agreement, and actionable recommendations.
Through these roles, METS Lab helps businesses in the food & beverage, water supply, packaging, and related industries ensure that their products are not only safe and compliant—but compelling to consumers. Sensory evaluation via METS Lab supports quality control, product development, brand reputation, and alignment with market and regulatory expectations in the Middle East and beyond.

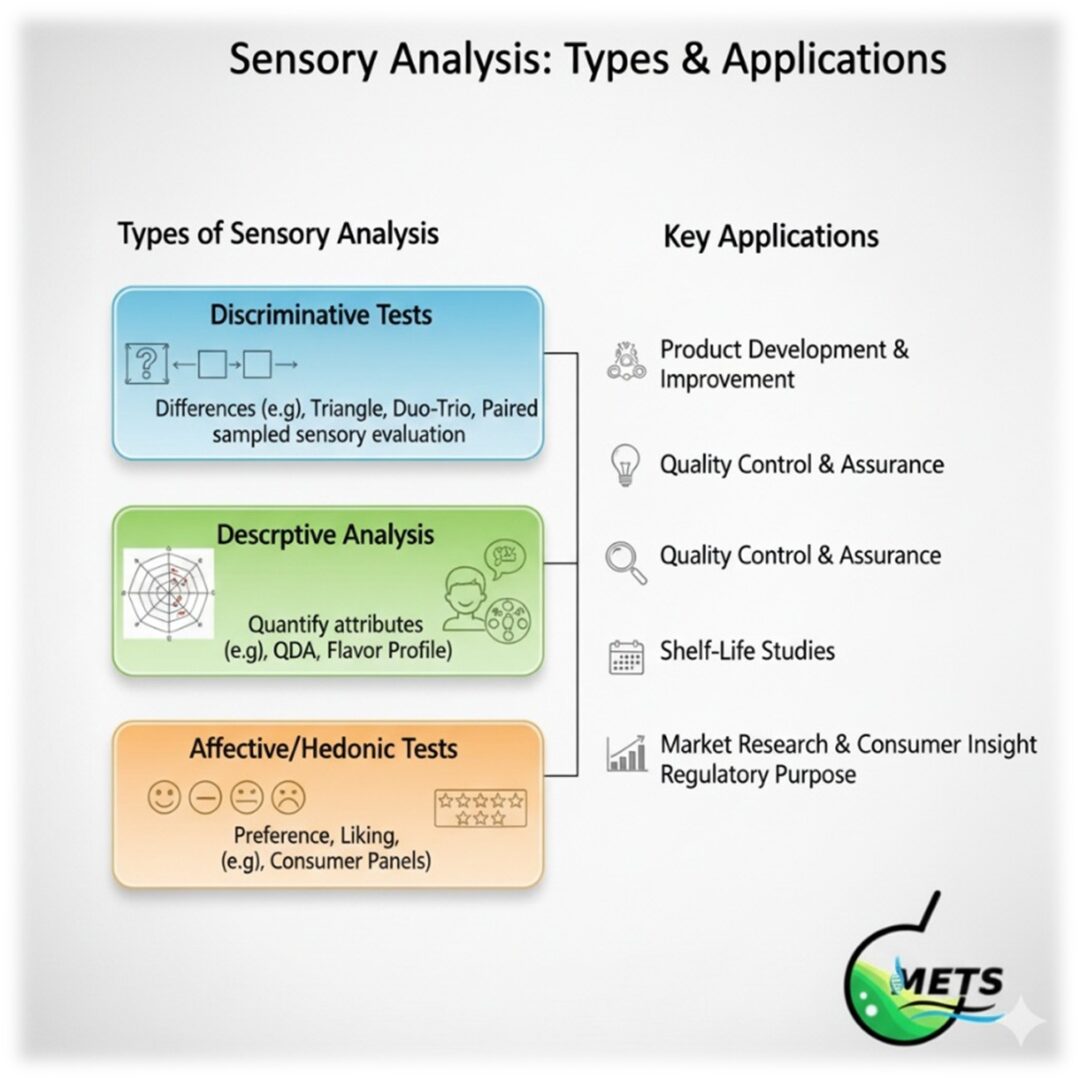
-Types of Sensory Evaluation Testing-
METS Lab provides a full range of sensory tests aligned with ISO standards:
- Difference Tests: Detect perceptible differences between products.
- Triangle Test: Participants are presented with three samples—two identical and one different—and must identify the odd sample. This test is effective for detecting subtle changes in products.
- Duo-Trio Test: A reference sample is provided, followed by two test samples. Participants determine which test sample matches the reference. This method is useful for identifying differences due to ingredient or process changes.
- Paired Comparison Test: Two samples are compared directly to assess which one is preferred or has a stronger attribute. This test is commonly used in consumer preference studies.
- Descriptive Tests: Quantify the intensity and quality of sensory attributes.
- Flavor Profile Method: A trained panel describes the flavor attributes of a product, including intensity, order of appearance, and aftertaste. This method provides a comprehensive flavor profile.
- Texture Profile Method: Panelists evaluate the texture attributes of a product, such as hardness, cohesiveness, and chewiness, to create a detailed texture profile.
- Spectrum Descriptive Analysis: Combines the Flavor and Texture Profile methods with rigorous training and statistical analysis to provide a detailed sensory profile.
- Free-Choice Profiling: Untrained consumers describe the sensory attributes of a product in their own terms, providing insights into consumer perceptions.
- Affective Tests: Measure consumer preference and acceptance.
- Paired Preference Test: Consumers are presented with two samples and asked to indicate which one they prefer. This test helps determine consumer preferences between products.
- Ranking Test: Consumers rank multiple samples in order of preference. This method provides a hierarchy of consumer preferences.
- Hedonic Scale: Consumers rate their liking of a product on a scale, typically ranging from “dislike extremely” to “like extremely.” This scale assesses overall consumer acceptance.
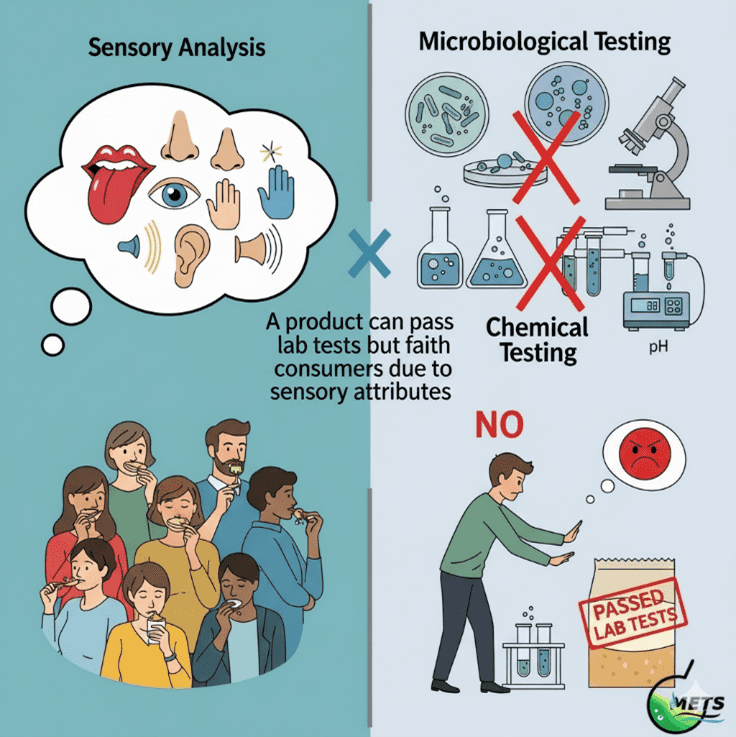
-Routine Testing Isn’t Always Enough To Ensure Quality-
Passing microbiological and chemical tests ensures safety—but does not guarantee consumer satisfaction. A product may meet all lab standards yet fail to delight the senses. Organoleptic testing bridges this gap by evaluating taste, odor, texture, and appearance, providing actionable insights to fine-tune formulations, enhance quality, protect your brand, and increase profitability.
-Products Suitable for Sensory Evaluation-
METS Lab has extensive experience analyzing a wide range of products:
| Category |
Examples |
| Water |
Bottled, mineral, flavored, carbonated |
| Baked Goods |
Bread, cakes, muffins, cookies, pastries |
| Canned Foods |
Seafood, vegetables, fruits, ready-to-eat meals |
| Coffee & Tea |
Beans, ground coffee, tea leaves, herbal teas |
| Dairy |
Milk, cheese, yogurt, butter, cream |
| Flour & Grains |
Wheat, rice, corn flours, pasta, breakfast cereals |
| Juices & Beverages |
Fruit/vegetable juices, soft drinks, smoothies |
| Meat & Seafood |
Beef, lamb, poultry, seafood, processed meats |
| Sauces & Condiments |
Ketchup, mayonnaise, dressings, soy sauce |
| Soups & Ready Meals |
Canned soups, instant noodles, frozen meals |
| Spices & Seasonings |
Ground/whole spices, herb blends, baking ingredients |
| Vegan & Plant-Based |
Burgers, sausages, meatballs, tofu, plant-based milk |
| Snacks & Confectionery |
Chocolates, candies, chips, nuts, energy bars |
| Frozen & Refrigerated |
Ice cream, frozen fruits/vegetables, frozen meals |
| Specialty Foods |
Fortified, nutritional shakes, gluten-free/keto, probiotics |

-How Organoleptic Properties Are Measured-
Sensory attributes are evaluated using a 5-point hedonic scale:
1 – Unacceptable
2 – Below Satisfactory
3 – Satisfactory
4 – Above Satisfactory
5 – Excellent
Attributes scored include: appearance, color, odor, texture, package integrity, pH, headspace analysis. This approach turns subjective perception into actionable data, enabling product improvement.
-Why Choose Middle East Testing Services (METS Lab)? –
Accredited and Compliant
Our laboratory adheres to international standards, including ISO 8586 (Selection and Training of Sensory Assessors), ISO 8589 (Design of Sensory Analysis Rooms), and ISO 13301 (Sensory Analysis Methodology). We implement stringent procedures to ensure the reliability and accuracy of our sensory evaluations, providing clients with trustworthy results that meet global quality and consumer expectations.
Expertise and Experience
METS boasts a team of skilled professionals with extensive experience in sensory evaluation. Our experts are well-versed in the complexities of sensory testing and can provide insightful analyses tailored to your specific products and requirements.

Comprehensive Reporting
We deliver detailed, easy-to-understand reports that include sensory profiles, consumer preferences, and any potential areas for product enhancement. Our comprehensive data helps clients make informed decisions regarding product design, market positioning, and regulatory compliance.
-Conclusion-
In today’s highly competitive food and beverage market, ensuring safety and meeting nutritional standards is no longer sufficient. Consumers demand products that not only meet regulatory requirements but also delight their senses. Sensory evaluation plays a critical role in assessing taste, aroma, texture, appearance, and overall acceptability, bridging the gap between product safety and consumer satisfaction. By leveraging rigorous sensory testing, brands can enhance product appeal, build lasting consumer trust, maintain consistent quality, and ultimately achieve market success.
At METS Lab, we combine state-of-the-art facilities, ISO/IEC 17025-accredited processes, and experienced sensory panels to provide actionable, data-driven insights. Our expertise ensures that your food and water products not only comply with standards but also deliver a superior sensory experience that resonates with consumers. Trust METS Lab to help your products stand out in the market through sensory excellence.