Spark Optical Emission Spectrometry
(Spark-OES)
Spark emission spectroscopy (OES) is an analytical technique employed in metallurgy and materials science for the determination of chemical composition of metallic substances. Spark OES will be a good choice for the quantitative evaluation of properties of numerous elements in less than one minute. As it provides accurate, precise, robust, stable and reliable results make this technique unique and vital in quality control as well as material testing. If you want to know more about this method then make sure to get in touch with METS Laboratories, Global.
How does spark emission spectroscopy work?
Spark OES analysis is accomplished by using a sparking process involves application of an electrical charger to the material sample resulting in vaporizing a small amount of material. The occurrence of spark causes a plasma discharge with a unique chemical signature which enables the experts for determining the sample’s elemental breakdown. The results obtained from the sample breakdown are in percentage of the constituent elements.
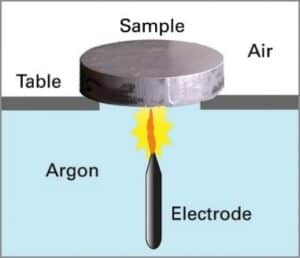
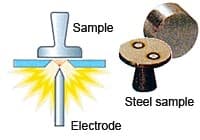
What is the procedure during analysis using spark emission spectroscopy?
Spark OES worked based on the principle of optical emission spectroscopy; during the analysis the sample will be ablated and excited in the argon plasma after the induction by an arc spark discharge. The sample emits light in the UV-Visible region based on the elements present in it or its concentration. The radiation emitted is transferred into the spectrometer via optical fiber where it is dispersed into its spectral components by the diffraction grating. The photons with wavelengths of elements of interest are detected and transformed into electrical signals with the aid of photomultiplier tubes or CCD detector/both into concentrations through calibration curves.
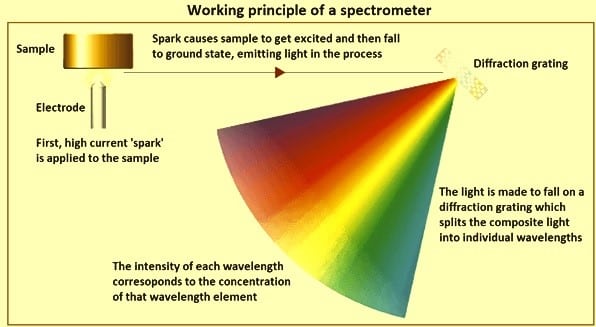
Spark Optical emission spectrometry (Spark-OES) a reliable tool for Positive Material Identification of steel alloys and non-ferro alloys
Spark-OES deliver ultrahigh speed of measurement, ultralow limits of detection, ultimate elemental flexibility, spectacular stability, and affordable cost of ownership makes it an unexceptional solution in metals industry, research and development (R&D), and process/quality control.
Advantages over Handheld EDXRF



Handheld X-ray fluorescence (XRF) analyzers are widely used across numerous industries and applications. However, XRF technology faces substantial limitations for nine elements due to the lack of sensitivity of the technology. The nine elements are carbon, sulfur, phosphorus, aluminum, silicon, magnesium, lithium, beryllium, and boron. Optical emission spectrometry with spark excitation allows a much more precise measurement.
Nine Elements That Challenge Handheld XRF Analyzers — but are easy for SPARK-OES

Determination of Lithium in Aluminum based alloys by Spark OES
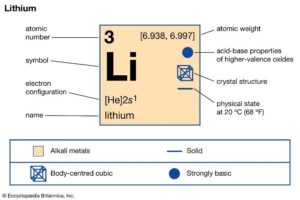
The lightest metal, lithium (Li) is frequently found in aluminum-based alloys. Incorporation of lithium decreases weight without affecting strength. Aluminum-Lithium finds use in aerospace and high-performance applications due to its high strength, low density, high stiffness, superior damage tolerance, excellent corrosion resistance, superior fatigue cracking resistance and weldability. The common lithium-aluminum alloys are 2099 and 8090 series. 8090 series comprised of 2.2% to 2.7% lithium, which are widely used in aerospace manufacturing. It is efficient enough to be an alternative to 2014 and 2024 aluminum alloys. 2099 is an aluminum lithium alloy, with low-density material with high stiffness. With superior damage tolerance and excellent corrosion resistance, the alloy offers excellent weldability. Several techniques are available to determine the exact concentration of alloys like ED-XRF, XRF, Spark OES etc. Among these Spark OES can be employed for the detection of Lithium like lighter elements in minute quantities (ppm level) where ED XRF failed to detect.
Determination of Beryllium in Copper based alloys by Spark OES
Beryllium Copper alloy is also known as Beryllium Bronze or Spring Copper, in which copper is alloyed with 0.5-3% Beryllium. It is generally used in cryogenic equipment due to its consistent strength, even at low temperatures. They can be classified as high strength copper beryllium and high conductivity copper beryllium. The category high strength copper beryllium has the highest strength of any copper alloy, and its tensile strength can exceed 200,000 psi. Beryllium Copper alloy efficiently transmits heat and electricity so it is commonly used for electronic connectors, computer components, and telecommunications products. Additionally, the alloy is non-sparking as well as it possesses nonmagnetic characteristics. This alloy can be either softened or hardened as needed by applying different heat treatment processes with extremely high corrosion resistance. Wires, electronic springs and connectors, oil and gas equipment components, automotive powertrain components, as well as undersea and marine telecom components are the major products manufactured from Beryllium Copper alloy. In spite of these advantages of Beryllium; it is among the chemical elements most toxic to humans. It should be mandatorily identified any beryllium content in a copper piece undergoing grinding in workshops, so that precautions can be taken to prevent exposure via skin or lungs. Beryllium is yet another element that’s impossible to measure effectively with handheld XRF spectrometers but can be effectively detected by Spark-OES.
Determination of Carbon in Steel via Spark OES
Carbon greatly influences the mechanical properties of carbon steel, including its mechanical strength, hardness, and ductility. Both stainless steel and carbon steel feature same basic composition, but carbon steel can be defined by its carbon content. The maximum carbon content in carbon steel is 2%. It can be categorised as low (carbon content less than 0.25%), medium (carbon content of 0.25-0.6%) and high carbon steel (carbon content from 0.6-1.25%). Steel alloys are weldable without special measures when they contain up to about 0.2% carbon, after that point, weldability decreases as carbon content increases. Type 316 stainless steels are widely used in the construction of petrochemical plants which contain chromium and 0.07% carbon. While 316L stainless steels contain “low carbon” contain a maximum of 0.03% carbon. Small difference even in ppm level of carbon is enough to give these alloys clearly different intergranular corrosion behaviors. Unlike handheld XRF instruments, Spark OES can easily differentiate these alloys on the spot.
Determination of Boron in low alloy steels by Spark OES
Boron is commonly added to steels to increase hardenability. Boron is added at a very low concentration of 0.0015 to 0.003 percent to increase the hardenability of low carbon steel. It enhances the cutting performance of high-speed blade steels. Low concentrations of boron in austenitic steels improve the high-temperature strength of the finished alloy. These steels are used as construction steels, and for screws and fasteners. It is not possible to analyze boron effectively with handheld XRF technology in which Spark OES take the advantage.
Determination of Other elements/alloys via Spark OES
|
|||||||||||||||||||
ConclusionSPARK-OES are optimized for use in metal
production, metal processing and metal recycling. Unlike conventional handheld instruments, SPARK-OES enables the precise analysis
of elements with low atomic
numbers such as C, P, S, B, Li, Be, Ca, Si, Mg, Al within a few seconds. Spark-OES can be a perfect
solution due to its ease, reliability and adaptability to use in every working
environment. |


